Young women can benefit from new tools for breast cancer detection
Syantra aims to raise awareness around risk of breast cancer in women under 50
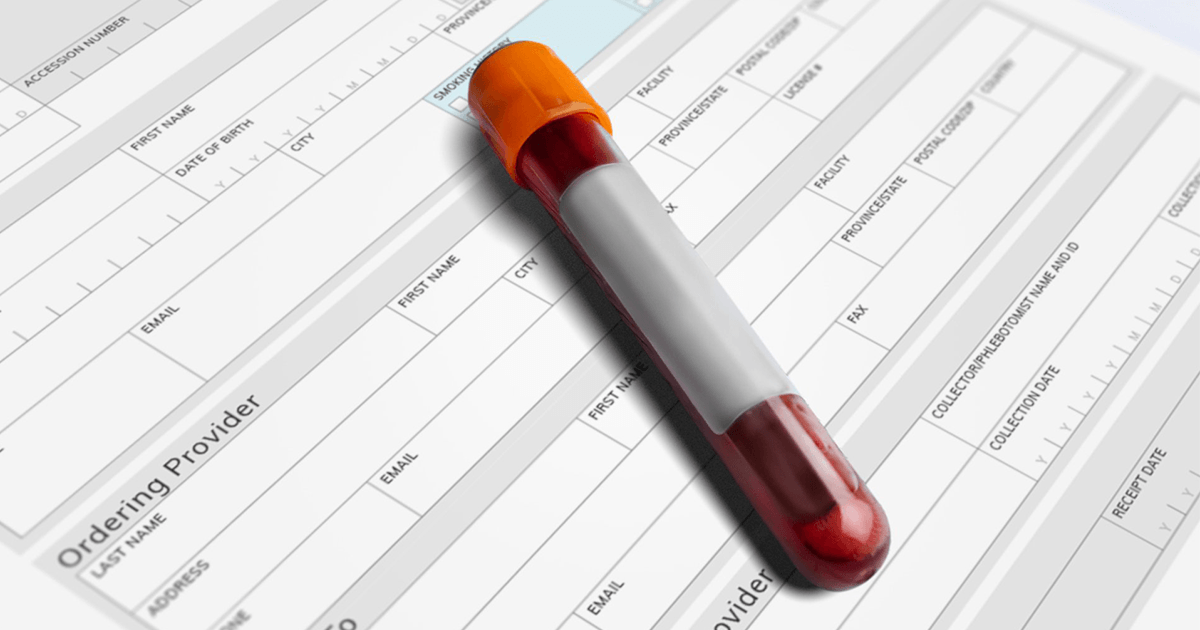
Calgary, AB. With the arrival of Breast Cancer Awareness month, Calgary precision biotechnology company, Syantra Inc., is announcing a program to help younger women expand their knowledge of breast cancer and screening. As part of this program, Syantra is launching the 18% Initiative to draw awareness to the percentage of breast cancer diagnoses in women under 50.
One out of eight women will develop breast cancer in her lifetime.¹ Half of women diagnosed will be under 65, with almost 20% being under 50.
“While women over 50 make up the majority of breast cancer diagnoses worldwide, younger women also need to be aware of the disease and understand their risk and screening options,” said Dr. Tina Rinker, Syantra co-founder and Chief Science Officer. “Many young women are not aware of their risk of developing breast cancer, in part because screening has historically not been recommended until age 50.”
Canadian breast cancer screening guidelines were changed in 2011 to recommend against regular screening in average risk women under 50. This topic has recently been in the news, and is sure to continue to generate debate. As the conversation continues, younger women can benefit from being informed about breast cancer and having options for testing. This may be particularly important for the roughly half of all women with dense or very dense breast tissue – a characteristic that’s more common among premenopausal women.
Syantra DX™ Breast Cancer is a private pay, physician requisitioned blood test to help identify breast cancer. It is based upon a suite of biomarkers and custom software to provide a positive or negative test result. This molecular approach provides another way to test for breast cancer that shows very good performance, with an overall accuracy of 98.5% in women under 50.² The test is offered through Syantra’s accredited laboratory in Calgary, and can detect invasive disease at early stages. A patient’s ordering physician will receive the test result and determine any necessary follow-up steps.
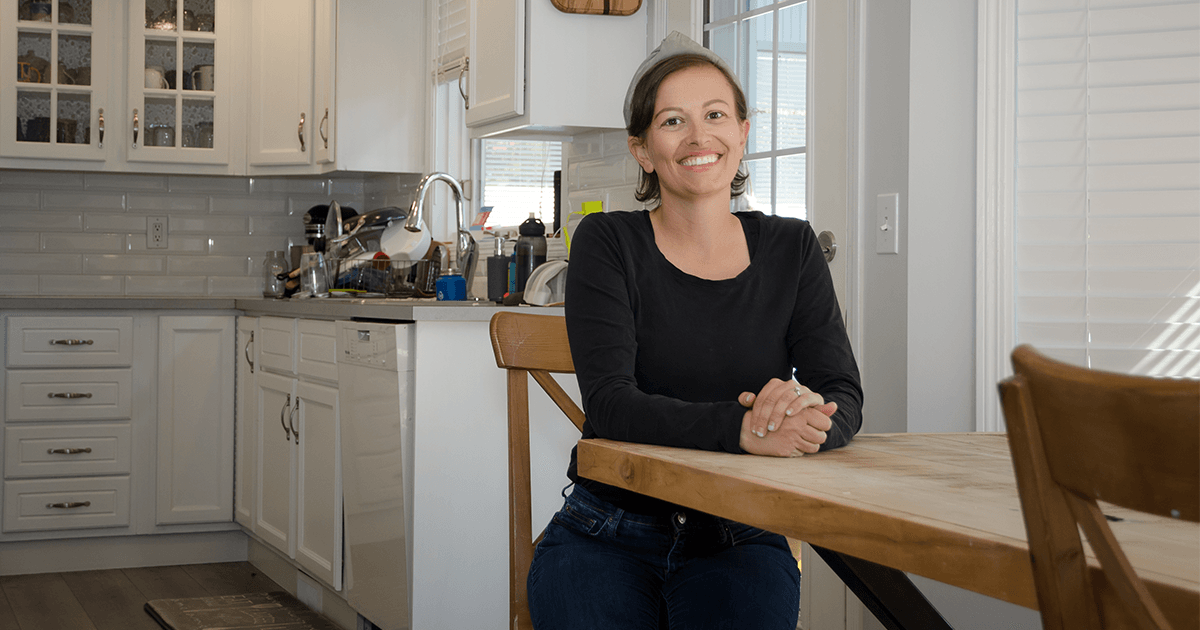
Jesslyn’s story
Jesslyn Davies understands first-hand the importance of awareness around breast health. In November 2019, Davies, a 33-year-old registered nurse and mother of two young boys, noticed a small cyst on her breast.
By Christmas time, Davies was diagnosed with Triple Negative Stage 2B breast cancer, a type of cancer that’s more common in younger women, tends to grow and spread faster, and has fewer treatment options. After responding well to the initial treatment, Davies’ cancer metastasized to her lungs. She is currently undergoing a second round of treatment, which she hopes will respond as well as her first round did.
“It seemed strange because cancer hasn't really affected anyone in my life,” said Davies. “I was young and always active and healthy. I felt like my risk factors were just not there. So you just wonder… why did this happen?”
While Davies is one of many women who may never get the answer to that question, she is certain that earlier detection would have improved her outcome.
“Early detection is so important, especially considering my breast cancer and how fast it grew,” said Davies.
In North America, 18 per cent of breast cancer diagnoses are among women under 50, before the typical age for breast cancer screening programs. Women under 50 tend to be detected at later stages, resulting in poorer outcomes. While the five-year survival rate for stage one breast cancer is approaching 100 per cent, the numbers for later stage diagnoses are sobering: for stage four diagnoses, the rate drops to only 22 per cent.³
Women under 50 have a 1 in 49 chance of developing breast cancer. We need better tools to enable expanded screening to young women and other underserved populations. “Young women can and do get breast cancer,” says Rinker “and current screening practices can create a gap in detection. We need new technology to expand the available tool kit, and Syantra is pleased to be able to offer a simple way to get tested.”
The 18% Initiative
In October, Syantra will launch an awareness campaign and a resource hub with information, tools, and connections to organizations active in breast cancer awareness and advocacy. More information is available at syantra.com/18percent.
¹SEER Cancer Statistics Review, 1975-2017, 2020.
²Syantra DX™ Breast Cancer has been evaluated in clinical studies for women between the ages of 25 and 80. The data infers an accuracy of 92.2% for women between 25 and 80, and 98.5% for women under 50. See IDBC study on clinicaltrials.gov: Bundred, N. et al., A whole blood assay to identify breast cancer: interim analysis of the international identify breast cancer (IDBC) study evidence supporting the Syantra DX Breast Cancer Test, San Antonio Breast Cancer Symposium, 21-A-1625-SABCS, presented December 8, 2021, San Antonio, TX, USA.
³Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2019 . Toronto, ON : Canadian Cancer Society; 2019
-30-
About Syantra Inc.
Syantra Inc. is a precision healthcare company that’s changing the way cancer is detected and treated. Its flagship product, Syantra DX | Breast Cancer, is a blood test for breast cancer identification that utilizes a proprietary suite of biomarkers and software to provide a positive or negative result.
For information or interviews:
Lisa Rushka, APR
Momentum Communications






2023 | Syantra Inc. | 1-877-331-0516
We value your privacy.
Read about it here
Website and branding by:
IVY Design Inc.
We